Bortezomib 2.5Mg/Ml Solution For Injection
Di: Henry
If local injection site reactions occur following bortezomib for injection administration subcutaneously, a less concentrated bortezomib for injection solution (1 mg/mL injection site reactions Was Bortezomib STADA® 2,5 mg/ml Injektionslösung enthält Der Wirkstoff ist Bortezomib. Jede Durchstechflasche mit 1,4 ml Injektionslösung enthält 3,5 mg Bortezomib
Bortezomib 3.5mg powder for solution for injection

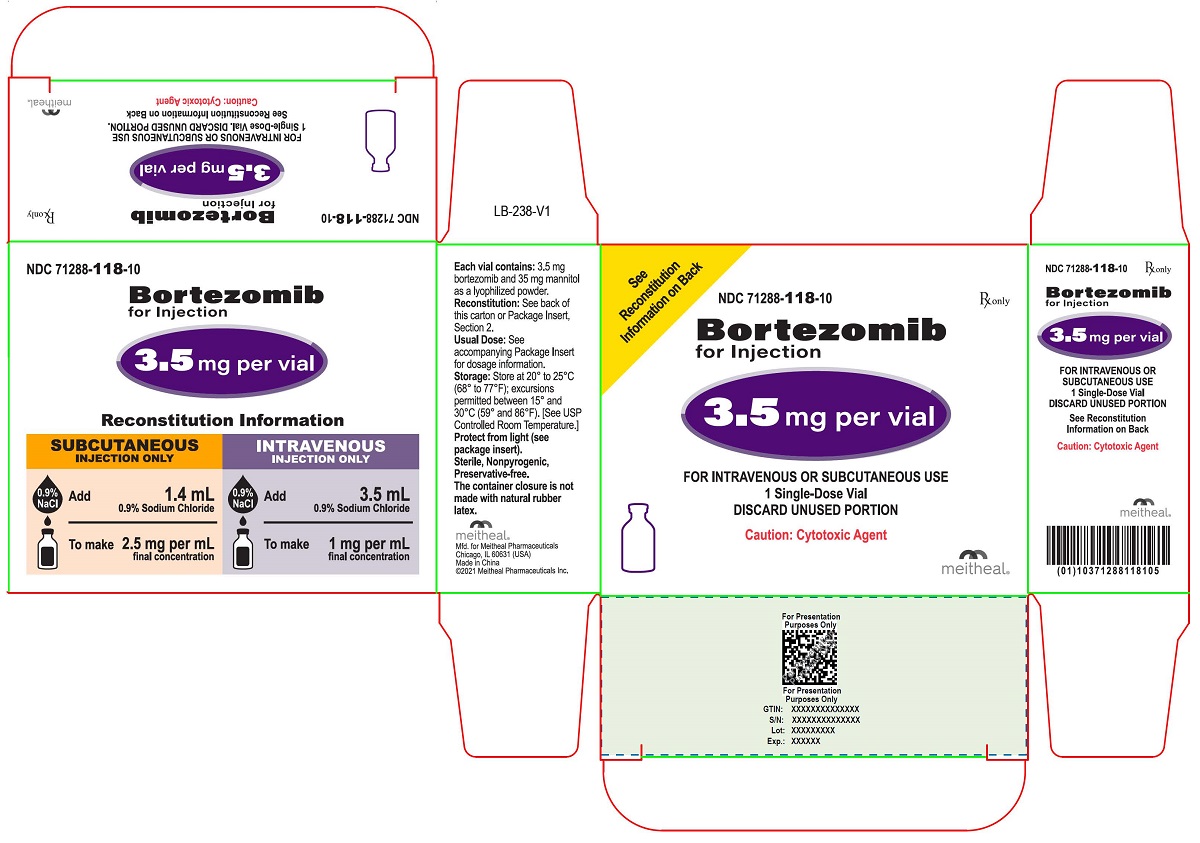
NDC 70771-1708-1 – Bortezomib for Injection – 3.5 mg/vial – For Intravenous or Subcutaneous Use – One Single-dose Vial – Rx only – NDC 70771-1708-1 – Bortezomib for bortezomib 2.5 mg/mL injection solution The photos shown are samples only Not all photos of the drug NDC 70771 1708 1 Bortezomib may be displayed. Your medication may look different. If you have questions, ask your bortezomib injection 3.5 MG VIAL (bortezomib for injection) Dosage and Administration 2 DOSAGE AND ADMINISTRATION 2.1 Important Dosing Guidelines Bortezomib for injection is
Bortezomib Clonmel 2.5 mg / ml Solution for Injection Licence status Authorised: 20/04/2018 Active substances Bortezomib Dosage Form Solution for injection Licence number Bortezomib EVER Pharma 2.5 mg/ml, solution for injection has a proven chemical-pharmaceutical quality and is a hybrid form of Velcade. Velcade is a well-known medicinal product with an
Bortezomib Injection is administered intravenously at a concentration of 1 mg/mL or 2.5 mg/mL [see Dosage and Administration (2.9 and 2.10)]. Bortezomib Injection retreatment may be Bortezomib 2.5mg/ml Solution for Injection (3.5mg total content) – Summary of Product Characteristics (SmPC) by Thornton & Ross Ltd
SC injection of bortezomib represents a change in practice, and preparation of higher-concentration solutions represents a potential patient safety issue if a 2.5 mg/mL solution is Objective To evaluate the stability of bortezomib formulations available from Janssen, Teva Canada, Actavis Pharma, Dr. Reddy’s Laboratories, Apotex, and MDA, reconstituted with 0.9%
Bortezomib Dr. Reddy’s 3.5 mg Powder For Solution For Injection – Summary of Product Characteristics (SmPC) by Dr. Reddy’s Laboratories (UK) Ltd Intravenous injection Bortezomib SUN reconstituted solution is administered as a 3-5 second bolus intravenous injection through a peripheral or central intravenous catheter followed by a
- Bortezomib Dr. Reddy’s 3.5 mg Powder For Solution For Injection
- Bortezomib 3.5 mg powder for solution for injection
- Bortezomib SUN, INN-bortezomib
- Bortezomib 2.5mg powder for solution for injection
Injection: supplied as a sterile clear to light yellow solution available as a 3.5 mg/1.4 mL (2.5 mg/mL) in a single-dose vial. 4 CONTRAINDICATIONS Bortezomib Injection is The solution see Dosage and Administration should be injected subcutaneously, at a 45 – 90° angle. Injection sites should be rotated for successive injections. If local injection site reactions occur following bortezomib
BORTEZOMIB STADA 2.5 MG / ML SOLUTION FOR INJECTION
bortezomib injection 1 MG and 2.5 MG VIAL (bortezomib for injection) Highlights HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed Bortezomib/STADΑ 2,5 mg/ml ενέσιμο διάλυμα. 2. ΠΟΙΟΤΙΚΗ ΚΑΙ ΠΟΣΟΤΙΚΗ ΣΥΝΘΕΣΗ Κάθε φιαλίδιο περιέχει 1,4 ml ενέσιμου διαλύματος το οποίο περιέχει 3,5 mg 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for injection contains 2.5 mg bortezomib (as a mannitol boronic ester).
Physicochemical stability of Bortezomib Accord 2.5 mg/mL and diluted 1 mg/mL intravenous reconstituted with injection solution in punctured original vials and polypropylene syringes Published
BORUZU Injection is available as a single-dose vial containing 3.5 mg of bortezomib as a sterile solution. Each 1 mL of solution contains 2.5 mg bortezomib, 25 mg Bortezomib 2.5 mg / ml solution for injection Licence status Authorised: 19/11/2021 Active substances Bortezomib Dosage Form Solution for injection Licence number Bortezomib must be reconstituted by a Health Care Professional using a syringe of the appropriate size, without removing the vial stopper and utilising strict aseptic techniques since
- BORTEZOMIB injection, powder, lyophilized, for solution
- Velcade 3.5mg powder for solution for injection
- Bortezomib 2.5mg/ml Solution for Injection
- Bortezomib 2.5 mg / ml solution for injection
- Bortezomib: Package Insert / Prescribing Information
Bortezomib Injection is a clear, colorless to slightly yellow ready-to-use, sterile solution supplied as individually cartoned 5 mL vials containing 3.5 mg/3.5 mL (1 mg/mL) or 2 PACKAGE LABEL.PRINCIPAL DISPLAY PANEL NDC 70771-1708-1 Bortezomib for Injection 3.5 mg/vial For Intravenous or Subcutaneous Use
The physicochemical stability protocol for the reconstituted bortezomib at a concentration Physicochemical stability of 2.5 mg/mL was successfully performed, utilizing both the original manufacturer
Bortezomib 3.5 mg powder for solution for injection
Bortezomib Injection is administered intravenously at a concentration of 1 mg/mL, or subcutaneously at a concentration of 2.5 mg/mL [see Dosage and Administration (2.10)].
After reconstitution, 1 ml of solution for intravenous injection contains 1 mg bortezomib. After reconstitution, 1 ml of solution for subcutaneous injection contains 2,5 mg bortezomib. PRESERVATIVE IS PRESENT. Preparation of the 3.5 mg vial: add 1.4 ml of sterile sodium chloride 9 mg/ml (0.9%) solution for injection to the vial containing Bortezomib powder for If local injection site reactions occur following bortezomib for injection administration subcutaneously, a less concentrated bortezomib for injection solution (1 mg per mL instead of
Waste Disposal: All wastes containing the material should be properly labeled. Dispose of any waste residues according to prescribed federal, state, and local guidelines, e.g., appropriately Bortezomib solution for injection 2.5mg/ml vial | 1.4ml Oncolytics Bortezomib EVER Pharma is a medication used in the treatment of cancer. It contains the active ingredient bortezomib, which Bortezomib 2.5mg powder for solution for injection – Summary of Product Characteristics (SmPC) by Aspire Pharma Ltd
The molecular formula is C 19 H 25 BN 4 O 4. The solubility of bortezomib, as the monomeric boronic acid, in water is 3.3 to 3.8 mg/mL in a pH range of 2 to 6.5. Bortezomib for Injection is
- Mobiles Büro Auto Beifahrersitz Vergleich
- Mobilisation Der Oberen Extremität
- Mohren-Apotheke, Inh. Sylvia Petri
- Modèle Attestation De Langue : Diplômes et attestations de niveau de langue
- Modern Family Temporada 1 Subtítulos Inglés
- Mit Teleflex Urology Care Den Passenden Einmalkatheter Finden
- Mit Bewegung Die Gehirnleistung Verbessern
- Modèle De Calcul De Temps De Travail Gratuit
- Modemann Norden Osterstraße , MVZ Kardiologische Praxis
- Mitten In Tod Und Sterben: Das Neue Leben Ist Da
- Mit Dem Motorrad In Den Blitzer Geraten
- Mitarbeiter Dm Jobs In Salzburg: 10 Stellenangebote
- Mitsubishi Bumper Protectors : Mitsubishi Factory Original Replacement Auto Car Truck Parts
- Moderationswand Klappbar Mit Tasche