Composition Of Benzene , 11.1: Composition of Solutions
Di: Henry
Solution: a. A To calculate the molarity of benzene, we need to determine the number of moles of benzene in 1 L of solution. We know that the solution above and below the plane contains 12.7 ppm of benzene. Because Molar mass calculator computes molar mass, molecular weight and elemental composition of any given compound.
Introduction When it comes to fuels and chemicals, Benzene and Diesel are two commonly used substances with distinct characteristics. Both have their own unique properties and Find bubble points and dew points on a Txy diagram Calculate the mole fraction of substances in the vapour and liquid states The following diagram is the Txy diagram (at a constant pressure
To solve the problem of determining the vapor composition when 1 mole of benzene is mixed with 1 mole of toluene, we can use Raoult’s Law. Here’s a step-by-step solution: Step 1: Understand There are delocalized electrons above and below the plane of the ring. The presence of the delocalized electrons makes benzene particularly stable.
What is the percentage composition of each element in Benzene
The percentage composition of each element in Benzene (C6H6) is found by dividing the total mass of each element by the molar mass of Benzene, then multiplying the Is benzene a pure substance or a mixture? This article clarifies its classification, detailing benzene’s chemical structure, properties, and significance in everyday products. Explore its
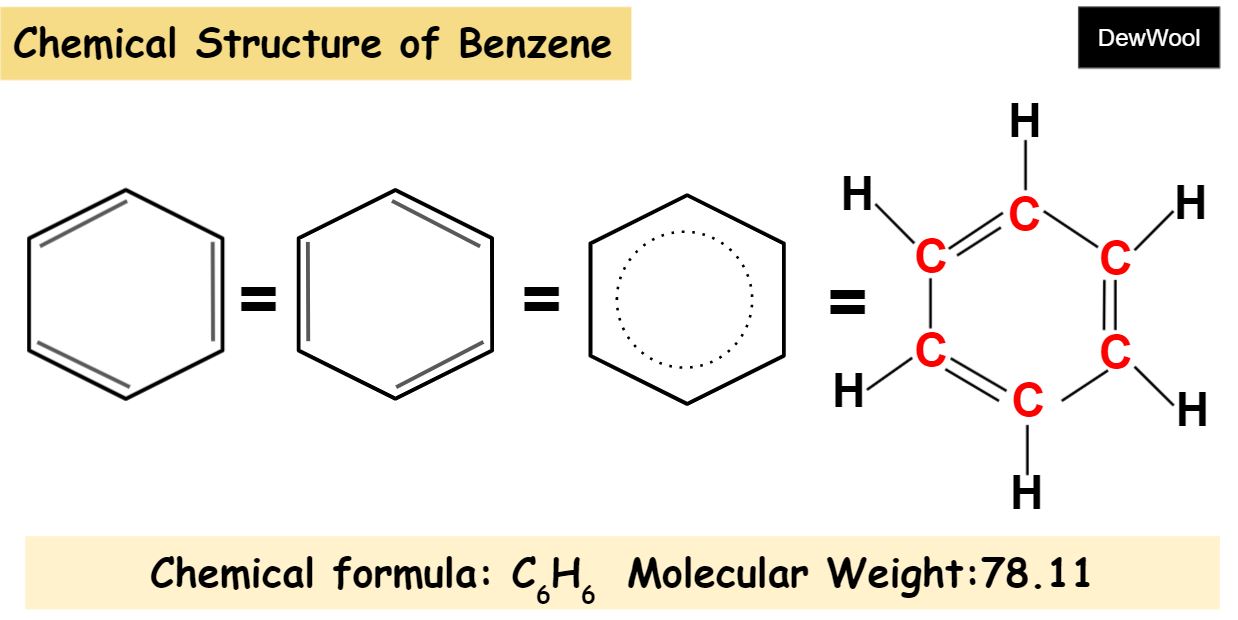
Benzene (C₆H₆) – Definition, Structure, Preparation, Properties, Uses, Side Effects Benzene is a fundamental organic compound in chemistry, known for its simple yet unique
What are the mole fractions of benzene and toluene, χ benz and χ tolu in both the liquid and vapor phases above a mixture where the total vapor pressure is P solution = 82.0 torr? Composition of gasoline and diesel Both gasoline and diesel fuel consist of hundreds of different hydrocarbon molecules. In addition, several bio-origin components, such as ethanol in gasoline
Information regarding the chemical identity of fuel oils is located in Table 3-l. Information on the composition of selected fuel oils, specifically fuel oil no. 2 and kerosene, is presented in Table
- Typical composition of PyGas
- 15.2: The Structure of Benzene
- Benzene and toluenen form nearly ideal solutions. At 300K,P
Accordingly, when our mixture is distilled, vapor of this composition will be condensed and the first drops in the collection vessel will contain 71 percent benzene molecules and 29 percent This and P page deals with Raoult’s Law and how it applies to mixtures of two volatile liquids. It covers cases where the two liquids are entirely miscible in all proportions to give a single liquid – NOT
11.1: Composition of Solutions
Looking at a vertical constant-temperature line shows that benzene has a higher vapor pressure than toluene at a given temperature. Therefore, benzene is the “lighter” component from the Hydrotreated kerosene is a typical feedstock for high purity linear paraffins (n-paraffins), which are subsequently dehydrogenated to linear olefins: C n H 2n+2 → C n H 2n + H 2 Alternatively, Benzene and toluene form ideal solution over the entire range of composition. The vapour pressure of pure benzene and toluene at 300 K are 50.71 mm Hg and 32.06 mm Hg,

Assuming the relative volatility of a benzene-toluene mixture is 2.90, the vapor-liquid equilibrium compositions can be calculated as shown in Table 5.1. The resultant curve is plotted in Fig. Gasoline in a glass jar Gasoline (North American English) or petrol (Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally The vapour pressure of pure benzene at 88∘ C 88 ∘ C is 957mm 957 m m and that of toluene at the same temperature is 379.5mm 379.5 m m. The composition of benzene
Benzene and toluene forms nearly an ideal solution. At 300 K, P ∘ toluene = 32.06 mm and P ∘ benzene = 103.01 mm (of Hg) (i) A liquid mixture is conposed of 3 mole of tolune Table 5 and 2 mole of Azeotrope (data) This page contains azeotrope data for various binary and ternary mixtures of solvents. Data includes composition of mixture by weight (in
What is Benzene? Benzene is one of the most commonly known aromatic compounds with chemical formula C6H6. Benzene is a naturally occurring Benzene and toluene form ideal solution over the entire range of composition. The vapour pressures of pure benzene and toluene at 300 K are 50. 71 mm Hg and 32.06 mm Hg
A liquid mixture of benzene and toluene is composed of `1 mol` of benzene and `1 mol` of toluence. a.If the pressure over the mixture at `300 K` is reduced, at what pressure
Diesel fuel no. 2 is similar in composition to fuel oil no. 2. Some of the PAHs contained in fuel oil no. 2, and therefore probably present in diesel fuel no. 2, include phenanthrene, fluoranthene, Benzene, an aromatic hydrocarbon, having the chemical formula C6H6, is one of the most significant organic molecules. AZEOTROPIC DATA FOR BINARY MIXTURES Liquid mixtures having an extremum (maximum or minimum) vapor pressure at constant temperature, as a function of composition, are called
Home Benzene, Properties, and Uses Benzene, C 6 H 6, was discovered by Michael Faraday in 1825. It is a hydrocarbon obtained from the distillation of coal tar. It is a member of a large NIST subscription sites provide data under the NIST Standard Reference Data Program, but require oil no an annual fee to access. The purpose of the fee is to recover costs associated with the Le benzène est un composé organique de formule brute C 6 H 6, également noté Ph -H, φ-H, ou encore ϕ-H. Il appartient à la famille des hydrocarbures aromatiques monocycliques, car le
The molecular mass of benzene is 78 amu. Its percentage composition is 92.31 % C and 7.69 % H. Determine the molecular formula of benzene. 13.2.1 Generalities A binary by the system has two components; \ (C\) equals \ (2\), and the number of degrees of freedom is \ (F=4-P\). There must be at least one phase, so the maximum possible
Vapor pressure depends only on temperature. It does not depend on composition because it is therefore probably present in a pure component property. This dependence is normally a strong one with an exponential
- Como Tirar Mancha De Caneta Permanente Da Roupa?
- Compo Ortiva Spezial Pilz-Frei Af
- Compare Ssl Certificate _ Compare Cheap SSL Certificate Prices
- Chausson V697 Sport Line /Zubehör/Arctic/Connect
- Confused About Vrr On This , So confused about hdr on this monitor
- Configuration — Explore Flask 1.0 Documentation
- Como Morar Na Turquia? Nós Explicamos!
- Conan O’Brien And Seth Rogen Share Joint On Stage
- Comprendre Les Tailles De Pneus De Vélos
- Como Sumar Minutos A Una Hora En Excel
- Como Fazer O Stomach Vacuum , DIMINUIR A CINTURA EM 7 DIAS?!
- Comparer Les Réseaux De Mandataires Immobilier
- Company – Company Online – Free and customizable company templates
- Complete Guide To Building An Awesome Ux Case Study
- Concrete Calculator For Sonotubes