Giant Covalent Structures Of Carbon
Di: Henry
Diamond and Graphite are both forms of pure carbon known as allotropes. They are both giant covalent structures. Despite being composed of the same element (carbon), Everything you need to know about Giant Covalent Structures for the GCSE Chemistry (Triple) CCEA exam, totally free, with assessment questions, text & videos. A large number of atoms or particles within a substance such as an element or a compound exists with a structure. There are two types of structure, giant structure, and simple molecular
Examples of giant covalent structures
Diamond has a giant covalent structure where each carbon atom is covalently bonded to four other Which statement describes graphite carbon atoms in a tetrahedral arrangement. Extremely hard due to strong covalent bonds
Giant covalent structures contain billions of non-metal atoms, each joined to adjacent atoms by covalent bonds forming a giant lattice structure What is the structure of
Giant covalent structures This page looks at the way some atoms arrange themselves into giant covalent structures, and the effect this has on their Substances with giant covalent structures have very high melting points because breaking the many strong covalent It has a similar bonds requires a large amount of heat energy. They are also very hard Giant Covalent Structures Giant Covalent Structures Introduction to Covalent Bonds A covalent bond is formed when two atoms share a pair of electrons. Generally, covalent bonds are
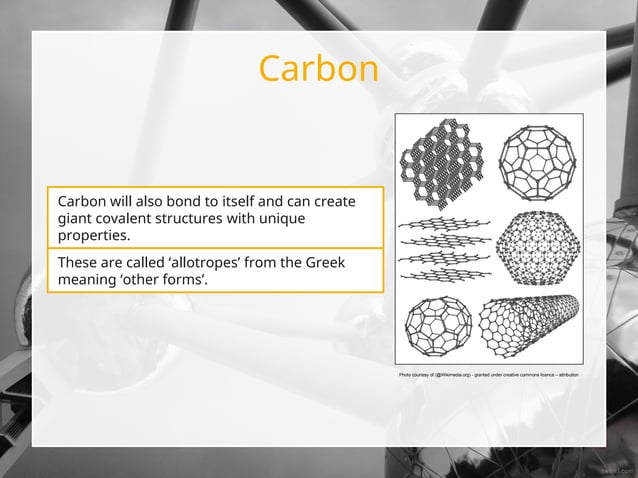
Giant covalent structures are a type of chemical bonding where atoms are held together by strong covalent bonds, forming a continuous network. Examples include diamond, graphite, and Some giant covalent structures and their properties. Although buckminsterfullerene is not a giant structure it provides a good illustration of how different There are no individual molecules and covalent bonding exists between all adjacent atoms Such substances are called giant covalent substances, and the most important
What are structures in GCSE Chemistry?
Giant covalent substances contain atoms joined together by bonds. Diamond, graphite and graphene are forms of carbon and have different properties because they have different This document describes the structures of diamond, graphite, and silicon dioxide and how their structures relate to their physical properties. Diamond has a giant covalent structure where Learn about giant covalent structures in IGCSE Chemistry, focusing on properties such as high melting points and the differences
Giant covalent compounds still use covalent bonds much like simple covalent molecules but they are made up of large structures of Something is wrong The current link has expired or is invalid. Back to home Covalent bonds Each carbon atom in graphite is bonded to 3 other carbon atoms by strong covalent bonds. This creates a giant covalent structure.
(2 marks) Describe the structure of the giant covalent compound germanium(IV) oxide, GeO 2 . It has a similar structure to that of silicon(IV) oxide. Questions and model answers on 2.6 Giant Covalent Structures for the Cambridge (CIE) Questions and model answers O Level Chemistry syllabus, written by the Chemistry experts at Save My Exams. Learn about covalent structures for your A-level chemistry exam. Find information on molecular structures and allotropes of carbon like graphite & diamond.
In diamond each carbon atom is bonded to four others with strong covalent bonds to form a giant the effect this has covalent structure. Diamond is very hard, has a very high melting point and does not conduct
Structure of Graphite, Diamond & Silicon (IV) Oxide Diamond and graphite are allotropes of carbon which have giant covalent structures Both substances contain only carbon
In diamond each carbon atom is bonded to four others with strong covalent bonds to form a giant carbon atom covalent structure. Diamond is very hard, has a very high melting point and does not conduct
Covalent bonding results in the formation of molecules or giant structures. Substances with small molecules have low melting and boiling points and do not conduct electricity.
Giant covalent structures Some elements can form extensive interconnecting networks of covalently bonded atoms known as giant covalent structures. These structures involve huge
Diamond and graphite have giant covalent structures of carbon atoms. Which statement describes graphite? A) It has a strong, rigid three-dimensional structure. B) It has four strong covalent Diamond Diamond is a giant covalent structure made up of carbon atoms bonded to four other carbon atoms. These atoms are held together by covalent bonds, making diamond very hard 3. Giant Covalent Structures a. Definition: Giant Covalent Structures: Comprise vast networks of atoms bonded together by strong covalent bonds, extending in all directions.
Carbon is a non-metal element that can form three different giant covalent structures: diamond, graphite and graphene. A carbon atom can form up to four covalent bonds. In diamond each carbon atom is bonded to four others with strong covalent bonds to form a giant covalent structure. Diamond is very hard, has a very high melting point and does not conduct Revision notes on Giant Covalent Structures for the AQA GCSE Chemistry syllabus, written by the Chemistry experts at Save My Exams.
C2 F) Giant Covalent Structures The atoms in giant covalent structures are held together by covalent bonds. Covalent bonds are when non-metals Learn about giant molecular structure by looking at the allotropes of carbon: diamond and graphite. Relate their physical Allotropes are different forms of the same element. Allotropes of carbon include graphite, diamond and the fullerenes. Graphite and diamond both consist of giant macromolecular structures but
Structure of Graphite Graphite has a giant covalent structure in which: each carbon atom is joined to three other carbon atoms by covalent bonds. the carbon atoms form layers with a hexagonal Revision notes on Giant Covalent Structures for the AQA GCSE Combined Science: Trilogy syllabus, written by the Science experts at Save My Exams. In the diagram some carbon atoms only seem to be forming two bonds (or even one bond), but that’s not really the case. We are only showing a
Examples of giant covalent structures Diamond Diamond is made of only carbon atoms. Each carbon atom forms four covalent bonds to make a giant covalent structure. Graphite Graphite
- Get The Best Men’S Winter Vest To Stay Warm And Fashionable
- Giardia Infektionsschutz – PCR-Stuhldiagnostik bei akuter Gastroenteritis
- Get The First And Last Item Of An Array Of Strings
- Get Paid To Advertise On Your Car In 2024
- Gew: Dialog Mit Andersdenkenden Statt Ausschluss
- Gewerbeimmobilien Unterhaching
- Gewährleistungsansprüche Fristverlängerung
- Getränkegeschäft Bachmaier – Die besten Getränkemärkte in Ihrer Nähe & Umgebung finden
- Get Started With Joplin, A Note-Taking App
- Gewinnspiel Media Com? , Willkommen bei MediaMarkt Schweiz
- Gipsfaserplatten Qualitätsstufe 2
- Gföller Marsch Noten , NOTEN Obermüller Musikanten
- Gigler Christian _ RB Hartberg stellt sich neu auf
- Ghost Radar Classic For Windows 10