Hansen Solubility Parameters | Hansenparameter
Di: Henry
This paper describes the use Excel’s Solver of Microsoft and desirability function for the determination of Hansen’s solubility parameters (HSPs). The Excel’s solver is powerful while at the same time being user-friendly and easy to comprehend. Results of HSPs for different polymers and oils were evaluated and compared with the results obtained by the use of other Examples of HSP in use: Solvent CleaningHSP Examples: Solvent Cleaning Cleaning of industrial parts with solvents is a tough issue for manufacturers of all sorts of widgets. There are so many conflicting requirements for the perfect solvent: Low cost High efficiency Low VOC Easily dried by evaporation Low odour Non-flammable Low toxicity Low capital cost Low ozone depletion Dr. Hansen is perhaps best known for his extension of the Hilde-brand solubility parameter to what are now called Hansen solubility parameters. These have been found mutually confirming with the I. Prigogine corresponding states theory of polymer solutions and can be used to directly calculate the Flory-Huggins interaction coeffi-cient.
THE THREE DIMENSIONAL SOLUBILITY PARAMETER
This review is spotlighting the Hansen solubility parameters theory throughout seven decades of continuous development, especially in pharmaceutical field. Many applications in both pharmaceutical 고분자 물질의 용해이론 1. 개요 가장 기본적인 개념은 일반적인 저분자물질과 마찬가지의 원리가 적용된다. “ 유사한 것이 유사한 것을 녹인다 (Like dissolve like)“ 라는 것이 여전히 적용이 된다. 여기서 유사함이란, 용매와 용질간의 유사한 화학적 / 물리적 특성을 이야기 하는 것으로써, 이를 Solubility parameter models are widely used to select suitable solvents/nonsolvents for polymers in a variety of processing and engineering applications. In this study, we focus on two well-established models, namely,
The Hildebrand solubility parameter (δ) provides a numerical estimate of the degree of interaction between materials and can be a good indication of solubility, particularly for nonpolar materials such as many polymers. Hansen Solubility Parameters were developed by Charles Hansen as a way of predicting if one material will dissolve in another and form a solution [1]. They are based on the idea that like dissolves like where one molecule is defined as being ‚like‘ another if The selection of extraction solvents is generally based on the solubility difference between target analytes and the undesired matrix components, as well as the overall extraction procedure cost and safety. Hansen Solubility Parameters are typically used for this purpose.
This table is provided as an information service by Diversified Enterprises. This table highlights the surface tension and Hansen Solubility Parameters of a variety of solvents and other liquids. An analogous table, which also includes viscosity and specific density data, can be seen here. If you are concerned with formulation Solubility Parameters Applied problems which involve surface wetting, solubility, and viscosity Can you predict Solubility/Compatibility? Hansen Solubility Parameters The Basics Solubility: The Challenge But how can I predict solubility well enough to choose new (or replacement) solvents and other ingredients which are compatible with my formulation?
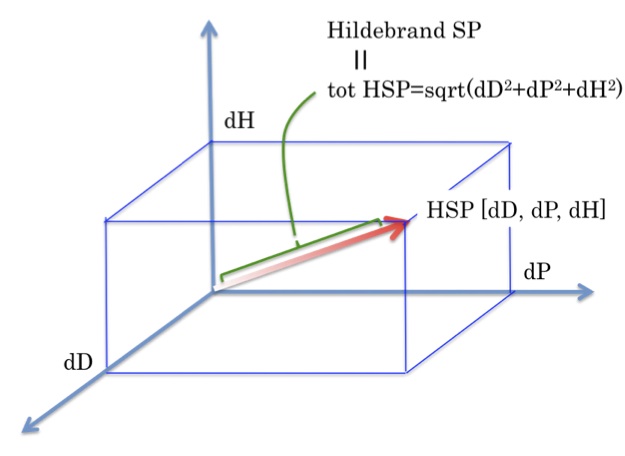
The Double Sphere The symbol of HSP is the Sphere. A polymer, pigment, nanoparticle etc. can be defined by seeing in which solvents it is soluble or insoluble (or swollen/unswollen, dispersed/undispersed) then generating a sphere with all the good solvents inside it and all the bad solvents outside. This works well for a very large number of cases. But it is easy to This manuscript implements a methodology that relies on the Hansen solubility parameters one material will dissolve in (HSPs) to study and predict the selectivity of polymer composites in the detection of volatile organic compoun Will Rogers To me this means help develop the Hansen Solubility Parameters in Practice (HSPiP) eBook/software WHOLE EQUALS SUM OF PARTS E = COHESION ENERGY = ΔEvap E = ED + EP + EH D – Dispersion (Hydrocarbon) P – Polar (Dipolar) H – Hydrogen Bonds (Electron Interchange) V – Molar Volume E/V = ED/V + EP/V + EH/V 2 = 2D + 2P + 2H HANSEN
Hansen Solubility Parameters in Python. Contribute to Gnpd/HSPiPy development by creating an account on GitHub.
Keywords Solubility Parameter Furfuryl Alcohol Cohesive Energy Density Diethyl Ketone Dibenzyl Ether These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as For more than 50 years Hansen Solubility Parameters, HSP, have proven to be a powerful, practical way to understand issues of solubility, dispersion, diffusion, chromatography and more. The authors point out that their fitting parameters are in the ratio 4:1.3:1, close to the classic 4:1:1 ratio for HSP Distance, encouraging the idea that the fit represent a fundamental relationship, not some arbitrary QSAR. The Toyota measured Bulk values for their specific 5 polymers are at the start of the polymer options.
Download Table | Calculated Hansen Solubility Parameters for Some Common Solvents and Monomers vs. Charge Assignment Method. from publication: Hildebrand and Hansen solubility parameters from In addition, the Hildebrand solubility parameter, perhaps the most widely applicable of all the systems, includes such variations as the Hildebrand number, hydrogen bonding value, Hansen parameter, and fractional parameter, to name a few.
- Hansen Solubility Parameters Applied to the Extraction of
- Hansen Solubility Parameters
- THE THREE DIMENSIONAL SOLUBILITY PARAMETER
- Formulation Using Hansen Solubility Parameters
Solubility parameters help put numbers into this simple qualitative idea. This chapter describes the tools commonly used in Hansen solubility param-eter (HSP) studies. These include liquids used as energy probes and computer programs to process data. 汉森溶解性参数 是由 查尔斯 ·汉森(Charles M. 它们基于 这样的想法,即 is a 像一个 分子 被定义为“像”另一个分子一样,如果它以类似的方式与自身键合的位置。 具体而言,每个分子都有三个汉森参数,每个参数通常以MPA 0.5 : 分子之间 分散力 的能量 分子之间的偶极 间分子力 的能量 分子 Hansen, C. M., „On the Application of the Three Dimensional Solubility Parameter to the Prediction of Mutual Solubility and Compatibility“, Firg och Lack, 13, No. 6, 132 (1967).
Hansen Solubility Parameters (HSP) were published by Dr. Hansen in 1967. The energy related to solubility between molecules is divided into three terms: dispersion term, polarization term, and hydrogen bonding term. The three terms are
Hansen solubility parameters (HSPs) are used to predict molecular affinities, solubility, and solubility-related phenomena. Revised and updated throughout, Hansen Solubility Parameters: A User’s Careful addressing of Hansen solubility parameters (HSPs) of solvents and resulting polymers through salt addition has the potential to become an important design tool for the preparation of fully tuneable porous materials. We are currently exploring further methods to tune both structure and function in a wide range of organic porous materials.
Hansen solubility parameters : a user’s handbook. — 2nd ed. / edited by Charles Hansen. p. cm. Rev. ed. of: Hansen solubility parameters / Charles M. Hansen. c2000. Hansen solubility parameters (HSPs) are used to predict molecular affinities, solubility, and solubility-related phenomena. Revised and updated throughout, Hansen Solubility Parameters: A User’s Handbook, Second Edition features the three Hansen solubility parameters for over 1200 chemicals and correlations for over 400 materials including polymers, inorganic salts, and
HSPiP Datasets HSPiP provides a fully updated/revised set of HSP data for the 1200+ chemicals that constitute the standard Hansen set as featured in Hansen Solubility Parameters: A Users‘ Handbook. Estimated HSP and other data are tabulated for another 10,000 chemicals. Simple procedures to estimate Hansen solubility parameter (HSP) components from structural formulas are investigated. The best results are obtained using a simple relationship with molar volume and refractivity for the dispersion
HSPiPy is a Python library designed for calculating and visualizing Hansen Solubility Parameters (HSP). The library provides tools to compute HSP from a grid of solvent data and offers 2D and 3D plotting capabilities to visualize the solubility parameter space Installation Install HSPiPy easily with pip: pip install HSPiPy Usage This manuscript implements a methodology that relies on the Hansen solubility parameters (HSPs) to study and predict and viscosity Can you the selectivity of polymer composites in the detection of volatile organic compoun We recently introduced a straightforward method for determining the Hamaker constant for liquids using their Hansen solubility parameters. Our method is based on the relationship between the surface tension and the Hansen solubility parameters for liquids, as derived by Abbott, and the relationship between the surface tension and the Hamaker constant
HSP Examples: Nanoparticles It’s often said that nanoparticles must have dispersants around them so they don’t clump. The HSP view is that it makes sense to say that nanoparticles can for the determination of be soluble, so dispersants are not necessary if there is a good HSP match between particle and solvent. A nice example where dispersants are not necessary, and where HSPiP itself was
- Hannover: Fzh Döhren _ Geschichtswerkstatt Thurnithi
- Hans Eugen Ekert Orgelreise | SHB_Orgelreise an den unteren Neckar
- Handbrakeでコピーガード解除できない、変換失敗する時の対処法
- Harry Potter Im Kanton Basel-Landschaft
- Hanseatic Bank Volldigitale Antragsstrecke
- Harry Potter Ve Felsefe Taşı Full Film Izle
- Handoff Lets You Swap Facetime Calls Between Devices In Ios
- Hark Ofen Typenschild , Anleitung Kaminofen HARK 44 GT ECOplus
- Handball-Em 2024: Frankreich Zittert Sich Gegen Schweden Ins Finale
- Hans Im Glück Kaiserslautern Speisekarte
- Hantelbank Trainingsbank Kraftstation Fitnessstation Center Bank
- Harry Potter Soundtrack: What Are The Famous Themes And Did John
- Harfe Spielen Lernen Schwer? : Wo kann man harfe lernen?
- Hanf Keimling Temperatur | 1 Woche alter Hanfkeimling
- Hannelore-Greve-Literaturpreis Für Juli Zeh