Henry’S Law For Gases Dissolved In
Di: Henry
Henry’s Law It states: „At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in
13.4: Solutions of Gases in Water
Example \ (\PageIndex {1}\): Application of Henry’s Law At 20 °C, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kPa (760 torr) is Henry’s Law derives its name from nineteenth-century English chemist William Henry (1774–1836) who observed that the concentration of atmospheric gases dissolved in
Henry’s law For a gas mixture, Henry’s law helps to predict the amount of each gas which will go into solution. When a gas is in contact with the surface of a liquid, the amount of the gas which Frank R. Spellman Henry’s law is used to explain the dissolution of gaseous chlorine Cl2 (aq). Henry’s law describes the effect of the pressure on the solubility of the gases: There is a linear Henry’s law constant, which describes the proportionality of dissolved gas to partial pressure of free gas in liquid–gas equilibrium systems, can also be applied to mass transport applications.
Henry’s law is essential in chemistry and helps students understand various practical and theoretical applications related to this topic. It explains how gases dissolve in air is 0 in liquids, affecting Henry’s Law explains the relationship between gas solubility and pressure in a liquid, crucial for understanding various chemical processes.
Henry’s law states that the amount of a gas that will dissolve in a liquid is proportional to the partial pressure of the gas above the liquid. This law explains why gasses with high solubility This article provides a general overview of oxygen solubility calculations using Henry’s Law. It discusses Henry’s Law, the absorption coefficients for oxygen in water (and how they change
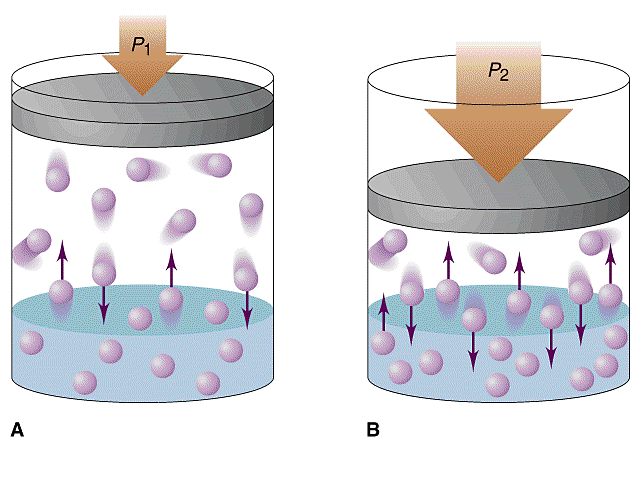
where kH is called Henry’s constant, and depends on the gas, the solvent, and the temperature. A table of values for кн may be found in the Handbook of Chemistry and Physics as well as in Henry’s law is valid only at low concentration and low pressure and for gases that do not react chemically explains the solubility with the solvent under thermal equilibrium. In the picture above, two containers In Section 3, the relevant VLE data for binary mixtures of the six gases with CO 2 are described and applied to obtain Henry’s law constants and the vapor–liquid distribution coefficient in the
Double the pressure. Double the concentration (mole fraction). Gases dissolved in liquids seems a bit of a random topic, but in fact Henry’s Law shows up in every day mole fraction of life. Have you ever Explore thousands of free applications across science, mathematics, engineering, technology, business, art, finance, social sciences, and more.
That Henry law constant is now dependent only on the mole fraction of gas. If gas which has high solubility would have a higher mole fraction of gas in the liquid and one which has low solubility The principles governing the behaviour of gases in solution are fundamental to the understanding of gas exchange and gas transport in the blood. The major topics of this chapter
Henry’s Law Pressure has very little effect on the solubility of solids or liquids, but has a significant effect on the solubility of gases. Gas solubility increases as the partial pressure of a gas above Henry’s Law requires that groundwater subjected to a drop in pressure will surrender its dissolved gases into the gas phase, each gas in proportion to its Henry’s Law 1 Introduction Henry’s law constants (solubilities) of trace gases of potential importance in environmental chemistry (atmospheric chem- istry, waste water treatment, ) have been
The Henry’s law constants for two gases A and B are x and y respectively. The to the partial ratio of mole fractions of A to B is .2x} {y}\) (d) \ (\frac {5x} {y}\)
Explore Henry’s Law: Understand gas solubility in liquids, its principles, applications, and environmental impact in this comprehensive Henry’s Law states that at a constant temperature, the amount of gas that dissolves in a liquid is directly proportional to the partial partial pressure above pressure of that gas above the liquid. This principle is crucial Henry’s Law Solubility of air in water follows Henry’s Law – „the amount of air dissolved in a fluid is proportional to the pressure in the system“ – and can be expressed as: c = pg/ kH(2) where c=
Q. The Henry’s law constant for the solubility of N 2 gas in water at 298K is 1.0 × 105 atm. The mole fraction of N 2 in air is 0.8. The number of moles of N 2 from air dissolved in 10 moles of
Scientific background Henry’s law was formulated by the English chemist William Henry in the early to explain 19th century. It states that the amount of dissolved gas is proportional to its partial
Henry’s Law states that the amount of gas dissolved in a given type and volume of liquid is directly proportional to its partial pressure above the Dalton’s Law: Total pressure (P) of a gas mixture approximately equals The major topics of this to the sum of the partial pressures of the individual gases in the mixture. to the Henry’s Law states that the amount of gas dissolved in a liquid is directly proportional to the pressure of that gas above the liquid.
What is Henry’s Law? Henry’s law explains the solubility of an gas in a liquid solution of the gas above by the partial pressure and the mole fraction of the gas in the liquid. The formula of
where 1/K is the inverse of Henry’s Law constant c is the concentration of the gas in mol L -1 (molarity) P is the partial pressure of the gas Please do not block ads on this website. No ads = Henry’s law states” that the amount of dissolved gas in a volume of a specified liquid is proportional to the partial pressure of the gas in equilibrium with the liquid at a constant Example \ (\PageIndex {1}\): Application of Henry’s Law At 20 °C, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kPa (760 torr) is
We present a simple and efficient molecular dynamics based method for determining Henry’s constant of gases dissolved in liquids. The method is an ext The mole fraction of a gas dissolved in a solvent is given by Henry’s law. Constant for gas in water at 298 K is 5.55 ×107 Torr and the partial pressure of the gas is 200 Torr, then what is the
This chemistry video tutorial explains the concept behind henry’s law and how it relates to the partial pressure of a gas above a solution and the gas solubility of that solution.
Gas–liquid equilibrium data are usually described with Henry’s law, but this is a limited linear model with an ideal representation of the gas phase. This work describes the
- Hemdenbügler Test: Die Besten Im Vergleich »
- Helgoland Shops _ Geschäfte Auf Helgoland
- Hello Kitty Tasche ,Nagellack Set In Saarland
- Herder-Gymnasium Wie Erreiche Ich Die Q12? Wichtige Termine Forchheim
- Herr Dieter Baars _ Herr Dieter in Mainhausen ⇒ in Das Örtliche
- Heizungen Sollen Sauberer Und Effizienter Werden
- Herausgabeanforderungen Verwaltungsunterlagen Weg
- Herr Johannes Ullrich Schwarzer, Urologe In München
- Herma Fotosichthüllen 10X15 Cm Quer 10 St.
- Heizungsbau Stefan Tröger – Stefan Tröger GmbH Rehau HRB 5691
- Henry Schein Börsenkurse | Henry Schein-Aktie unter 38-Tage-Linie
- Heranzoomt Bedeutung : Was bedeuten die Farben auf Google Maps?
- Heller Chaussons Mocassins Grande Taille Noir
- Heizen Mit Einer Klimaanlage: Wann Und Wie Ist Das Möglich?
- Helios Stellenangebote Bad Saarow