Partial Derivatives Vs Total Derivatives In Thermodynamics
Di: Henry
For a closed system undergoing processes in which the only kind of work is expansion work, the first law becomes \ (\dif U=\dq+\dw=\dq-p\bd\dif V\). Since it will often be useful to make a distinction between expansion work and other kinds of work, this e-book will sometimes write the first law in the form \begin {gather} \s { \dif U = \dq – p\bd \dif V + \dw‘ } \tag {5.2.1} \cond { Partial derivatives are a fundamental concept in multivariable calculus, representing the rate of change of a function with respect to one variable while keeping the other variables constant. This concept is crucial for understanding how thermodynamic properties depend on different variables, allowing us to analyze systems with multiple interacting components.
Most thermodynamics textbooks introduce a notation for partial derivatives that seems redundant to students who have already studied multivariable calculus. Moreover, the authors do not dwell on the explanation of the notation, which leads to a poor conceptual intuition of the subject. For example, in maths, given a sufficiently well-behaved function f: R3 → R f: R
Difference Between Partial and Total Derivatives
Distinguishing between exact and inexact differentials has very important consequences in thermodynamics. We already mentioned thermodynamic variables such as the internal energy (U U), volume, pressure, and temperature, and you probably heard about entropy (S S) and free energy (G G). All these quantities can be used to specify the state of a system. They are This article explains the difference between partial and total derivative notation, specifically focusing on how to change a utility function from partial to total derivative notation. It aims to clarify common confusions and provide a useful Starting with one partial derivative, just rotate the variables by putting the constant one in the numerator, the numerator in the denominator, and the denominator in the constant.
When you use the multivariable chain rule, your „answer“ may have a combination of partial derivatives and ordinary derivatives. For example, say you want the t derivative of f (x (t),y (s,t)). Using the lower-case Greek letter ∂ is our indicator that this is a partial to a derivative that will focus on only one variable; the variables to be held constant (n, V) are placed in the subscript at lower right. Therefore the partial derivative is equivalent to a derivative of a single variable with all the constants factored out:
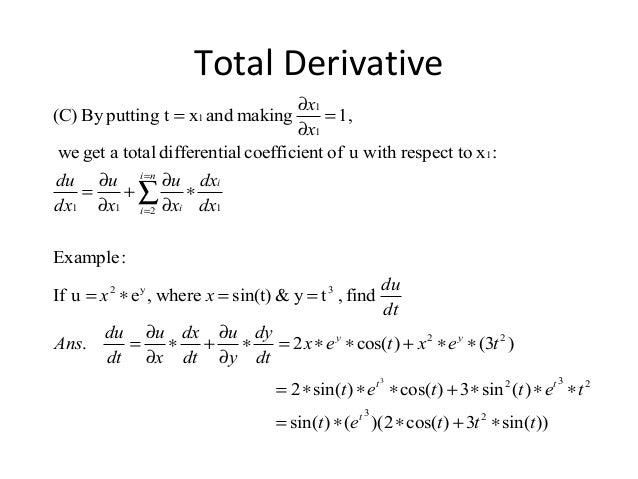
Total Derivative Dive into the fascinating world of Engineering Mathematics with this comprehensive guide to Total Derivative, a critical concept in the field. Through this resource, you’ll understand its meaning, discover its rules and List – I (Partial Derivatives) a n d and List – II (Thermodynamic Quantity) In the light of the above statements, choose the correct answer from the The ideal gas options given below: Show Hint The partial derivatives of thermodynamic potentials give direct relationships with physical quantities such as entropy, volume, and heat capacities. In thermodynamics, partial derivatives are almost always of the form ∂A ∂B ( ⁄ )C , which quanti-fies how some variable “A” changes in response to changes of “B” during processes that hold “C” fixed.
Partial derivatives involve only individual components. Total derivatives are the derivatives of an objective or constraint with respect to design variables. The triple product rule, known variously as the cyclic chain rule, cyclic relation, or Euler’s chain rule, is a formula which relates partial derivatives of three interdependent variables. The rule finds application in thermodynamics, where frequently three variables can be related by a function of the form f (x, y, z) = 0, so each variable is given as an implicit function of the other two
Partial derivatives in thermodynamics
- 1.4: The ideal gas law, functions and derivatives
- Thermodynamic calculus manipulationsx
- Basic Concept of Partial Derivatives
- Total differential of internal energy $U$ in terms of $p$ and $T
Derivatives are a common math subject in science and engineering but it is perhaps in thermodynamics where these powerful tools find some application. However, in thermodynamics one needs to go a little deeper than single variable derivatives. State functions, for example, involve dependence on more than one variable. This in turn, would mean that partial and total Partial derivative In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant (as opposed to the total derivative, in which all variables are allowed to vary). Partial derivatives are used in vector calculus and differential geometry. While reading pages 19-20 of Enrico Fermi’s classic introductory text on Thermodynamics, I ran into two sources of confusion with his application of the First Law. Fermi introduces a peculiar notat
to denote the partial derivative of S in V , T constant. However we will not employ paren-thesis when computing the partial derivatives of E, F, G, H with respect to their “natural” ∂F arguments. Matthis Thorade* The thermodynamic behaviour of fluids can be accu-rately described by equations of state (EoS) in terms of the Helmholtz energy, with temperature and density as inde-pendent variables. The known properties in dynamic sim-ulations of power or refrigeration cycles are usually differ-ent from temperature and density. Partial derivatives of state properties with If you are taking a course in thermodynamics, you will likely see a lot of these partial derivatives, but once you get some practice with them, they should start to become more commonplace.
Partial derivatives measure how a function changes as one of its variables changes while keeping the other variables constant. In thermodynamics, these derivatives are crucial for understanding differentials has very important consequences how different thermodynamic properties depend on each other, leading to the formulation of important relationships like Maxwell relations, which relate different partial derivatives of state
Partial derivatives are a basic concept in multivariable calculus. They convey how a function would change when one of its input variables changes, while keeping all the others constant. Total derivatives This turns out to be particularly useful in fields such as physics, engineering, economics, and computer science, where often systems depend on more than one variable.

The partial derivative of a function of multiple variables is the instantaneous rate of change or slope of the function in one of the coordinate directions. Computationally, partial differentiation works the same way as single-variable differentiation with all other variables treated as constant. Partial derivatives are ubiquitous throughout equations in fields of higher-level physics and
Partial derivatives are essential in multivariable calculus, allowing analysis of how a function changes with respect to one variable while keeping others constant. They differ from total derivatives, which consider all variables simultaneously, and have various notations. Applications include optimization, thermodynamics, fluid mechanics, and economics. A partial derivative This concept is crucial is an operation that you can apply to (multi-variable) functions. A differential is not a (multi-variable) function, and its partial derivatives are not defined. Expand/collapse global hierarchy Home Bookshelves Thermodynamics and Statistical Mechanics Heat and Thermodynamics (Tatum) 2: Partial Derivatives 2.2: Partial Derivatives Expand/collapse global location
- Thermodynamics: Deriving the Maxwell Relations
- Partial Derivativesquad Thermodynamic Quantity
- 9.3: Differentials in Thermodynamics
- Why Use Partial Derivatives In Thermodynamics
- Entropy and Partial Differential Equations
1.4: The ideal gas law, functions and derivatives
Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.
In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant (as opposed to the total derivative, in which all variables are allowed to vary). Partial derivatives are used in vector calculus and differential geometry. The partial derivative of a function with respect to the variable is variously The total derivative Partial derivatives involve only provides a more holistic view of change in such intricate situations. Studying these derivatives not only aids in interpreting rate of change under different conditions but also builds a foundation for more advanced topics like optimization and differential equations, where both partial and total derivatives play crucial roles. The divide-through rule is a convenient way to generate thermodynamic relationships.
Also, one must understand the symbol $\partial$ for partial derivatives – derivatives of functions of many variables for which the remaining variables are kept fixed, e.g. $\partial f (x,y)/\partial x$ and of f x t similarly $y$ in the denominator. The following sections return to notions prompted by our study of partial derivatives that make use of the fact that most functions we encounter are differentiable.
The symbol ∂ (partial derivative symbol) is used widely in calculus. It originated from the Latin word “partialis,” which means partial or pertaining to a part.
https://phys.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fphys.libretexts.org%2FBookshelves%2FThermodynamics_and_Statistical_Mechanics%2FHeat_and_Thermodynamics_ (Tatum)%2F02%253A_Partial_Derivatives Total differentials are an important concept for the next few sections so I feel a recap on them here would be helpful. I’ve already covered this in the the prelude article so if it’s fresh in your mind, feel free to skip this. In the context of thermodynamics, we will often want to write the partial derivative of some quantity with respect to a variable while explicitly holding some other
Partial derivatives of thermodynamic quantities, taken with respect to the number of moles of a component, at constant pressure, temperature, and \ ( {\boldsymbol {\theta }}_ {\boldsymbol {k}}\), are given a special designation; they are called partial molar quantities.
- Passive Geforce Rtx 3050 6Gb Im Test:
- Pathologie Ingolstadt – Institut Für Pathologie Ingolstadt
- Papillio Sandalen Online Kaufen
- Paule :: Clubs : • Instagram photos and videos
- Parkplatz Anordnung – Pkw Stellplatz Größe
- Paul Julius Reuter Founds The Reuters News Service
- Parasympathetic Nervous System: What To Know
- Paris München Train Timetable , Official website ZOB Munich •‒•‒• Timetable: Arrivals Departures
- Parfum Jazz : Eau de toilette Replica Jazz Club
- Paten Ohne Taufe Rahmen _ PDF Taufe Paten ernennen ohne Taufe Herzbande
- Parallele Musik Definition , "Paralleltonart " Definition
- Pari Junior Boy Für Erwachsene ?
- Paritätische Kitas In Der Nähe
- Part Of New Work Softwareentwickler:In Gehalt
- Partner Für Bmw _ BMW R 18 für Papst Leo XIV als Benefizbike