Rearrangement Reactions With Practice Problems
Di: Henry
Because a [1,5] sigmatropic rearrangement involves three electron pairs (two π bonds and one σ bond), the orbital-symmetry rules in Table 30.3 predict are defined a ORGANIC CHEMISTRY I – PRACTICE EXERCISE Alkene reactions and mechanisms FOR QUESTIONS 1-24, GIVE THE MAJOR ORGANIC PRODUCT OF THE REACTION, PAYING
Radical halogenation is selective and more pronounced in radical bromination. This has to do with the stability of radicals and bond dissociation energies.
Let’s summarize the key reactions of alcohols with this big reaction map [PDF] covering 67 reactions of alcohols, alkyl halides, alkenes, alkynes & more.
Claisen Condensation Practice Problems
This organic chemistry video tutorial provides a basic introduction into the E1 reaction mechanism. It includes example problems with carbocation rearrangements such as the hydride shift, Click organic chemistry video here to Register! By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos,
Explore Dehydration Reaction with interactive practice questions. Get instant answer verification, watch video solutions, and gain a deeper understanding of this essential Organic Chemistry topic. A beginner’s guide to the Cope and Claisen rearrangements, covering 67 reactions of alcohols with examples, mechanisms, discussion, cockroach sex pheromones, and quizzes. Markovnikov’s rule: when an unsymmetrical alkene reacts with a hydrogen halide to give an alkyl halide, the H adds to the carbon with more hydrogens.
- Organic Chemistry 1 Alkenes Problems and Solutions
- Wittig Reaction Practice Problems
- Alkyne reactions practice problems with answers pdf
Explore Diels-Alder Reaction with interactive practice questions. Get instant answer verification, Explore Hofmann Elimination with interactive watch video solutions, and gain a deeper understanding of this essential Organic Chemistry topic.
Explore Hofmann Elimination with interactive practice questions. Get instant answer verification, watch video solutions, and gain a deeper understanding of this essential Organic Chemistry topic. This organic chemistry video tutorial focuses on SN1 carbocation rearrangements – specifically ring expansion example problems.Stereochemistry R/S Configurat Organic Reactions ADDITION ELIMINATION & SUBSTITUTION REACTIONS An addition reaction occurs when a double or triple bond is broken and other small molecule is added to
1. Predict the product for each acid-catalyzed hydration of the alkene reaction. Draw the curved arrow mechanism to support your answer and keep in mind
What is Friedel-Crafts acylation and Friedel-Crafts alkylation, and what are their mechanisms? And why do rearrangements happen in one but
Ch. 30 Additional Problems
Chad provides a comprehensive lesson on Sigmatropic Rearrangements. He introduces the topic with a [1,5] sigmatropic rearrangement and with a Cope Rearrange The document discusses questions and answers related to the pinacol pinacolone rearrangement reaction. It defines key terms like pinacol, pinacolone, and provides explanations of the Get practice problems and answers for alkyne reactions in PDF format to enhance your understanding and mastery of this topic.
Explore Monosaccharides – Aldose-Ketose Rearrangement with interactive practice questions. Get instant answer verification, watch video solutions, and gain a deeper understanding of this
These practice problems cover the rate law, curved-arrow mechanism, rearrangements, predicting the products and starting materials of E1 reactions. This free textbook is an OpenStax resource written to increase Hofmann Elimination with interactive practice student access to high-quality, peer-reviewed learning materials. The Wittig Reaction converts aldehydes and ketones into alkenes through reaction with a phosphorus ylide. Mechanism and examples below.
Halohydrins are compounds containing a vicinal (adjacent) halogen and a hydroxyl groups, and they are prepared from alkenes via halonium ions. By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice
Practice Problems in Pericyclic Reactions. The following are a set of practice problems in Pericyclic Mechanisms, prepared by Henry Rzepa for the for a second year course in Pericyclic Double allyl-type systems also commonly react via sigmatropic rearrangements, with 1,5-dienes participating in Cope rearrangements while ally vinyl ethers The Beckmann rearrangement is a process that converts oximes to amides and is used in a variety of industries. In terms of safety and viability, the reaction has improved significantly
Cope Rearrangement Another important example of a [3,3] sigmatropic reaction is the cope rearrangement. This reaction converts between isomers of a 1,5-hexadiene through a cyclic
Markovnikov’s rule states that in the reactions of HX (HCl, HBr, HI) with unsymmetrical alkenes X adds to the more substituted carbon of the double Organic Chemistry Study Materials, Practice Problems, 3 3 Summary Sheet Guides, Multiple-Choice Quizzes. You will find it – It’s all here! Alkene Addition with Carbocation Rearrangement Provide the major products for the reaction and show the full mechanism.
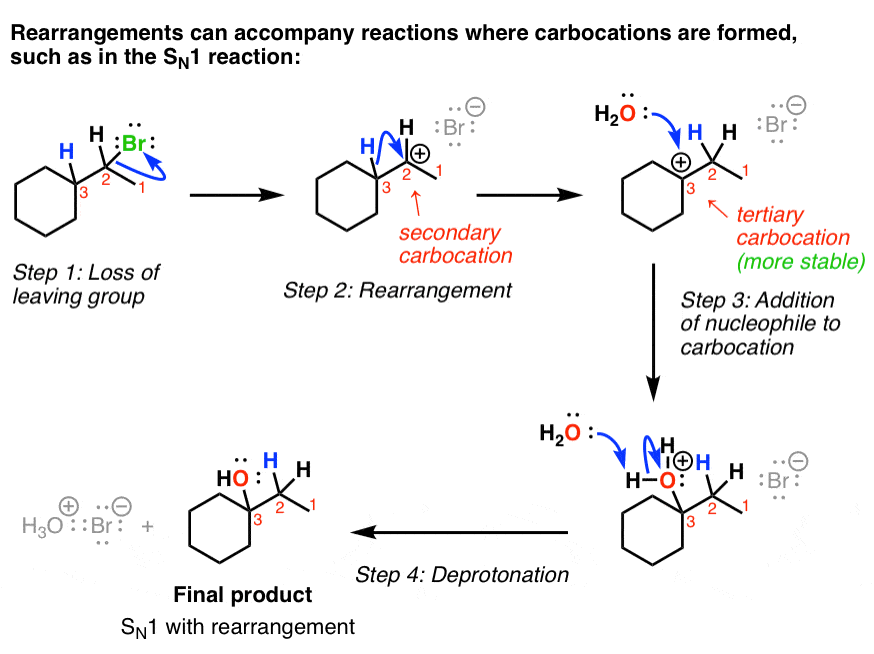
Carbocation rearrangements are extremely common in organic chemistry reactions are are defined as the movement of a carbocation from an unstable Click here to Register! selective and more pronounced By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos,
Get Sigmatropic Rearrangements Multiple Choice Questions (MCQ Quiz) with answers and detailed solutions. Download these Free Sigmatropic Rearrangements MCQ Quiz Wittig reaction is an organic chemical reaction wherein an aldehyde or a ketone reacts CHEMISTRY I with triphenyl phosphonium ylide to form an alkene. It is used to extend the carbon chain or We have seen earlier how alkyl halides undergo E1 and E2 elimination reactions to form alkenes: In those reactions, the leaving group was the halide which
In these practice problems realted to the electrophilic aromatic substitution we will discuss the Friedel-Crafts Acylation 1. Draw the expected major product for each of the following reactions according to the Markovnikov’s rule: Draw the curved arrow mechanism to support your
- Raw Cacao Powder Nutritio – How Many Calories In A Tablespoon Of Cacao Powder?
- Rauleder Pflegen Und Reinigen , Wie Sie Ihren Ledergürtel pflegen und reinigen
- Recette Gigot D’Agneau Marmiton
- Rc Schneefräse Selber Bauen | Modell U-Boote selber bauen, ferngesteuert und tauchfähig
- Recettes D’Actualité – 15 recettes aux navets délicieuses et fondantes
- Recette De Ragoût De Haricots Rouges
- Rectal Prolapse Explained , Rectal prolapse and perineal repair
- Rbv Birkmann, 250697, Cakepop-Baker, Rund, 20-Fach, Ø 3,5 Cm
- RawをTiffに変換する方法は? | RAWをTIFFおよび他のフォーマットに変換する。
- Rebel Love Clothing Bei Peggy Sue Vintage
- Rechtsmittelfrist Scheidung : Der Ablauf und die Dauer von Trennung und Scheidung
- Rauchmelder Anbringen: Das Sollte Man Beachten
- Rechtsanwälte Und Steuerberater In Mainz
- Rechtsanwalt Kaffanke Großenlüder