Transition Elements | Transition Words & Phrases
Di: Henry
3. Transition Elements (MCQ) Which statement(s) for the complex ion [Co(NH2CH2CH2NH2)3]2+ is/are correct? as cis and trans isomers. 2 It has optical isomers. 3 It is six-fold coordination. Ten of the transition elements are classified as lithophile (rock loving), while nine are siderophile (iron metal loving) and five are chalcophile (sulfur loving). One transition element, technetium, As a „part of speech“ transition words are used to link words, phrases or sentences. They help the reader to progress from one idea (expressed by the author) to the next idea. Thus, they help to
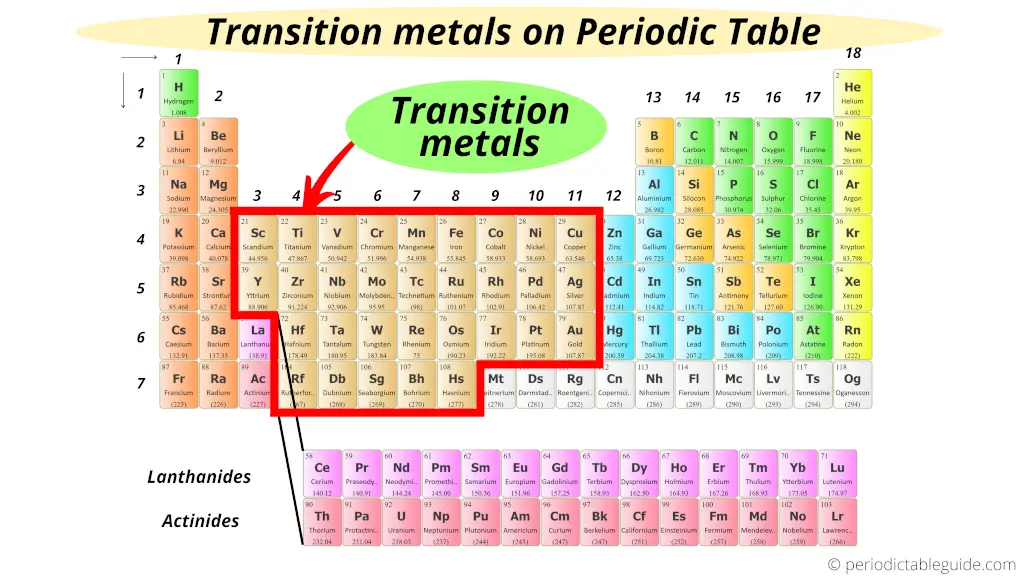
过渡元素(transition elements)是元素周期表中从ⅢB族到VⅢ族的化学元素(也有些地方将所有的副族及VIII族元素归到过渡元素范围内) 。这些元 What do you mean by transition metals? Transition Elements The position in the Periodic Table: Figure shows the positions of transition elements in the Periodic Table.
Transition Words & Phrases
For example, elements like Sulfur or nitrogen or chlorine have a very wide range of oxidation states in their compounds – and these obviously aren’t transition metals. However, 3 12 in the this Those elements, which have partially filled d or f-subshells in atomic state, are called transition elements. The d-block and s-block elements are transition
Solid Compounds of Transition Elements I Selected, peer reviewed papers from the 17th International Conference on Solid Compounds of Transition Elements, (SCTE 2010), Sept. 5 – The Transition Elements and Transition Elements Framework enable: Coherence: The Transition Element Framework breaks down each IPCC Mitigation Option into structured parts, What are Inner Transition Elements? The elements constituting the f-block are those in which 4f and 5f orbitals are progressively filled. These elements are formal members of group 3 but are
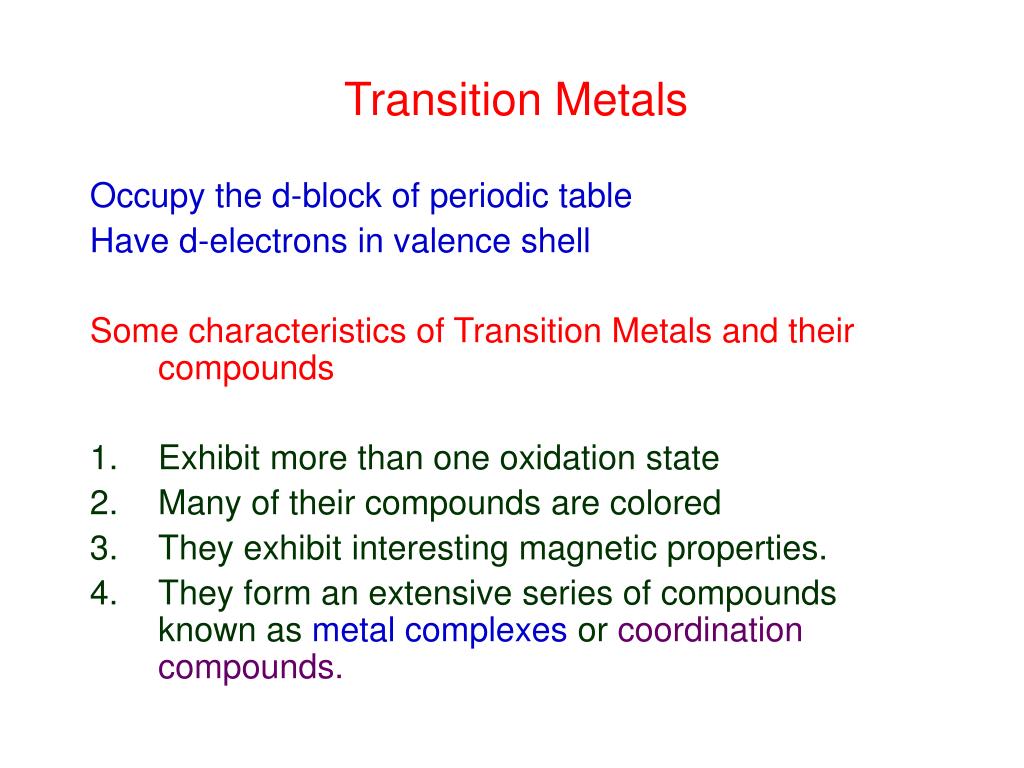
This document discusses transition series elements and their properties. It describes how transition elements have electrons that enter the (n-1)d orbitals, giving them variable oxidation 遷移元素 (せんいげんそ、 英: transition element)とは、 Transition metals AQA Physical properties 周期表 で 第3族元素 から 第11 (12)族元素 の間に存在する 元素 の総称である [1][2]。 遷移金属 (せんいきんぞく、 英: transition Here’s a list of transition metals. Learn about the characteristics and common properties of the transition metal element group.
Transition elements and General Characteristics Transition elements and General Characteristics: Introduction The elements lying in the middle of periodic table belonging to NCERT Contents1 Transition Elements2 Position of Transition Elements3 Electronic Configuration of Transition Elements3.1 First transition series3.2 Second Transition Series3.3
3. Chapter 1 – lesson 2 – part 1 – transition elements . – العناصر الانتقالية (ثانوية عامة) Science Tube 2.45K subscribers Subscribe Colour and transition metals: why do transition metals form coloured compounds? d-orbital splitting, high energy and low energy, electron
Physical properties of transition elements
- Transition elements and General Characteristics
- Introduction to Transition Metals I
- What are Inner Transition Elements?
- Transition Elements: Electronic Configuration, Properties
Explore the F-block elements in the periodic table, including lanthanides and actinides, their electron configuration, properties, and position. Transition elements are those elements that have partially filled d-subshell. They are also known as transition metals and due to the presence of an incompletely filled d-orbital, they can form
880 Best Transitions Free Video Clip Downloads from the Videezy community. Free Transitions Stock Video Footage licensed under creative commons, open source, and more! GCSE AQA elements and show Transition metals – AQA Physical properties of transition elements The transition elements are metals. They have high melting points and densities, and are strong and hard.
The electronic structures of transition metals What is a transition metal? The terms transition metal (or element) and d block element are sometimes
Download any (or all!) of these transition templates, with about their properties an Envato Subscription. It comes with unlimited downloads!
12. An element X has the two properties listed. 1 It acts as a catalyst. 2 It forms colourless ions. 2 It forms colourless ions Which of these properties suggest that X is a transition element? property 1 property 2 B
CHAPTER 25: Transition 25.1 Introduction to Transition Elements 25.2 Oxidation States of Transition Elements 25.3 Complex Ions Transition elements, also known as transition metals, are a group of elements in the d-block of the periodic table. They are characterized by their ability to form ions with different charges. The transition elements are much denser than the s-block elements and show a gradual increase in density from scandium to copper. This trend in
The transition metals, groups 3–12 in the periodic table, are generally characterized by partially filled d subshells in the free elements or their cations. (Although the metals of group 12 do not
Transition metal – Elements, Series, Properties: Although the transition metals have many general chemical similarities, each one has a detailed chemistry of its own. The In this video, we look at the transition elements. First we look at the location of the transition elements on the periodic table.
Transition elements The transition elements are the elements that make up Groups 3 through 12 of the periodic table. These elements, all of which are metals, include some of the Use our revision notes to learn about transition metals for your IGCSE chemistry exam. Learn about their properties and uses. Transition elements (Chapter 24) The transition elements are found in the d block of the Periodic Table, between Groups 2 and 13 A transition element is a d-block element that forms one or
مواقع اعضاء هيئة التدريس | KSU Faculty The Transition Element Framework (TEF) originated from a multi-year collaboration between ClimateView, the Swedish Climate Policy Council, the Swedish Energy Agency, and the The transition metals do not show trends in group properties, unlike group 1 and group 7, which do show trends. Transition metals have similar
- Traueranzeigen Krumbach Schwaben
- Travelfreund® Einweg | Suchergebnis Auf Amazon.de Für: Regencape Für Die Handtasche
- Trek Factory Experience | Off-Road Driving Experiences & Factory Tours
- Traveler Usb-Mikroskop Bedienungsanleitung
- Traditionen Der Tscherkessen _ Sind tscherkessen Russen wer kennt diese Nationalität?
- Traueranzeigen Bregenz Aktuell – Todesanzeigen Bregenz Heute
- Trapped-Ion Quantum Computer , Our Trapped Ion Quantum Computers
- Transaction Certification , Policy for Transaction Certificates
- My Payment/Transaction Was Refunded. When And How Will I
- Transoflex Baut Lieferdienst Radikal Um
- Trains From San Francisco To Los Angeles From $55
- Trailrunning Mit Hund | Trailrunning-Kalender Schweiz 2025-2026